 Eichrom’s TRU Resin is an extraction chromatographic material containing octylphenyl-N,N-di-isobutyl carbamoylphosphine oxide (abbreviated CMPO) dissolved in tri-n-butyl phosphate (TBP). The CMPO molecule is shown in figure 1. The bed density of TRU Resin is approximately 0.37 g/mL, with a working capacity of 2 mg Am per mL of resin or 4 mg Am per 2mL pre-packed column. This value represents 20% of the theoretical maximum loading capacity of the resin.
Eichrom’s TRU Resin is an extraction chromatographic material containing octylphenyl-N,N-di-isobutyl carbamoylphosphine oxide (abbreviated CMPO) dissolved in tri-n-butyl phosphate (TBP). The CMPO molecule is shown in figure 1. The bed density of TRU Resin is approximately 0.37 g/mL, with a working capacity of 2 mg Am per mL of resin or 4 mg Am per 2mL pre-packed column. This value represents 20% of the theoretical maximum loading capacity of the resin.
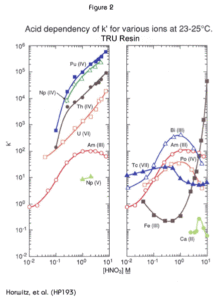 Figure 2 shows the uptake of various actinide elements from nitric acid solutions by the TRU Resin. The y-axis, k’, is a measure of uptake, corresponding to the number of free column volumes (FCV) to peak maximum in a chromatographic column. FCV is a measure of the interstitial space (or void volume) of a column. Values higher than 100 indicate strong uptake, values between 1 and 50 indicate weak uptake. The data presented here was generated by Horwitz, et al. at Argonne National Laboratory using experimental batches of TRU Resin. Eichrom’s commercial product conforms to established specifications that ensure proper performance of Eichrom issued methods. Please refer to our product specifications for details.
Figure 2 shows the uptake of various actinide elements from nitric acid solutions by the TRU Resin. The y-axis, k’, is a measure of uptake, corresponding to the number of free column volumes (FCV) to peak maximum in a chromatographic column. FCV is a measure of the interstitial space (or void volume) of a column. Values higher than 100 indicate strong uptake, values between 1 and 50 indicate weak uptake. The data presented here was generated by Horwitz, et al. at Argonne National Laboratory using experimental batches of TRU Resin. Eichrom’s commercial product conforms to established specifications that ensure proper performance of Eichrom issued methods. Please refer to our product specifications for details.
 The tetravalent actinides show extremely high retention on the column, with k’ in the range of 10E4-10E6 at HNO3 concentrations in excess of 2M. Hexavalent uranium is approximately one order of magnitude lower. The k’ curve for trivalent americium plateaus at about 100 FCV in the range of 1-5M nitric acid. It is important to note that trivalent plutonium behaves similarly to americium and that pentavalent neptunium exhibits very low retention at any nitrate concentration.
The tetravalent actinides show extremely high retention on the column, with k’ in the range of 10E4-10E6 at HNO3 concentrations in excess of 2M. Hexavalent uranium is approximately one order of magnitude lower. The k’ curve for trivalent americium plateaus at about 100 FCV in the range of 1-5M nitric acid. It is important to note that trivalent plutonium behaves similarly to americium and that pentavalent neptunium exhibits very low retention at any nitrate concentration.
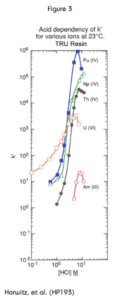
Figure 3 shows the retention behavior of the actinides in HCl. The very low affinity of the column for Am in chloride media forms the basis for the selective stripping of Am (and trivalent plutonium) from the TRU Resin.
Figures 4 through 6 show the effect of a number of matrix components on the uptake of the actinides on TRU Resin. 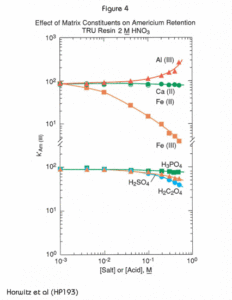 The effects of various cations and complexing anions on the retention volume (k’) of americium in 2M nitric acid on the TRU Resin are plotted in Figure 4. Among the cations shown, calcium and divalent iron show no effect on americium retention. This is particularly valuable since both calcium and iron are present in large quantities in many environmental and bioassay samples. They are often added as carriers in precipitation steps. Trivalent iron shows a significant, negative effect on Am retention. If it is suspected that iron is present in a sample, a reducing agent such as ascorbic acid should be added to ensure that all Fe(III) is reduced to Fe(II). Ammonium thiocyanate can be used as an indicator for this; it is a deep red color in the presence of Fe(III) and colorless in the presence of Fe(II). The effect of Fe(III) on Am retention will increase at higher HNO3 concentrations, where Fe(III) is more strongly retained.
The effects of various cations and complexing anions on the retention volume (k’) of americium in 2M nitric acid on the TRU Resin are plotted in Figure 4. Among the cations shown, calcium and divalent iron show no effect on americium retention. This is particularly valuable since both calcium and iron are present in large quantities in many environmental and bioassay samples. They are often added as carriers in precipitation steps. Trivalent iron shows a significant, negative effect on Am retention. If it is suspected that iron is present in a sample, a reducing agent such as ascorbic acid should be added to ensure that all Fe(III) is reduced to Fe(II). Ammonium thiocyanate can be used as an indicator for this; it is a deep red color in the presence of Fe(III) and colorless in the presence of Fe(II). The effect of Fe(III) on Am retention will increase at higher HNO3 concentrations, where Fe(III) is more strongly retained.
Addition of aluminum(III) increases the uptake of Am on the TRU Resin column. This happens because the aluminum (III) cation is readily hydrated in solution. This has the effect of increasing the activity of nitrate ions in solutions, driving the formation of the americium-nitrato complex that is readily extracted by the CMPO/TBP extractant system.
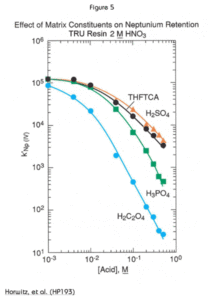
 As shown in Figures 4 and 5, the commonly occurring polyatomic anions show a relatively small effect on americium retention on the TRU Resin, but have a significant adverse effect on the retention of the tetravalent actinides. While Figure 5 shows only the effects on the retention of neptunium, the behavior of the other tetravalent actinides under the same conditions is analogous. The curve for plutonium is nearly identical to the neptunium curve; that for thorium would be parallel, but approximately one order of magnitude lower.
As shown in Figures 4 and 5, the commonly occurring polyatomic anions show a relatively small effect on americium retention on the TRU Resin, but have a significant adverse effect on the retention of the tetravalent actinides. While Figure 5 shows only the effects on the retention of neptunium, the behavior of the other tetravalent actinides under the same conditions is analogous. The curve for plutonium is nearly identical to the neptunium curve; that for thorium would be parallel, but approximately one order of magnitude lower.
Fortunately, the retention of neptunium and plutonium is high enough that even relatively large concentrations of sulfate and phosphate (up to 0.5M) should not cause breakthrough under the conditions indicated in our published methods. If thorium is being separated on TRU Resin, care should be taken for samples with elevated levels of phosphate or sulfate. The addition of aluminum nitrate will reduce the matrix effect as phosphate will complex the aluminum preferentially leaving thorium less affected.
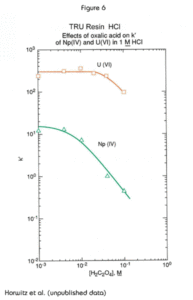
As shown in Figure 6, the effect of oxalate is quite significant for the tetravalent actinides, which readily form oxalato complexes that are not extracted by the CMPO/TBP solvent system. Uranium retention is not affected significantly by oxalate up to 0.1M. . For this reason, solutions of oxalate salts can be used to strip tetravalent actinides selectively from the TRU Resin without eluting uranium.
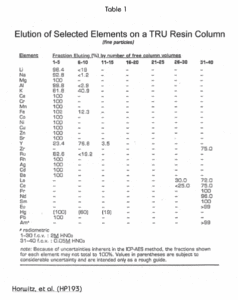 Table 1 lists the elution behavior of a number of commonly occurring elements on the TRU Resin in 2M nitric acid (FCV 1-30) and in 0.05M nitric acid (FCV 31-40.)
Table 1 lists the elution behavior of a number of commonly occurring elements on the TRU Resin in 2M nitric acid (FCV 1-30) and in 0.05M nitric acid (FCV 31-40.)
SOURCE for all published data: E. P. Horwitz, R. Chiarizia, M. L. Dietz, H. Diamond, and D. Nelson, “Separation and Preconcentration of Actinides from Acidic Media by Extraction Chromatography,” Analytica Chimica Acta, 281 (1993) 361-372. (HP193)
TRU Resin is manufactured in three particle sizes (20-50 µm, 50-100 µm, and 100-150 µm) and is sold in bottles or ready to use in prepackaged columns (for gravity flow) and cartridges (for vacuum assisted flow.) Click here for part numbers and descriptions.