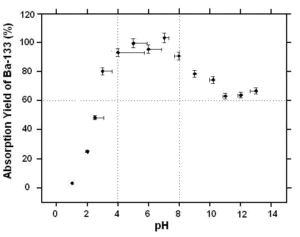
Figure 1: Absorption recovery in Ba-133 on MnO2 resin. 10mL deionized water, 25 mg of resin, magnetic stirring for 60 minutes at 20°C, pH adjusted with HCl or NaOH. Measurement done with a NaI well-type detector.
MnO2 Resin is useful for the separation of Ra from water samples. (Moon, et al. [1])
Different parameters such as pH, reaction time, resin quantity, salt effect and flow rate have been evaluated when separating Ra from water using MnO2. For most of the experiments, Ba-133 was used as tracer for Ra.
MnO2 Resin shows the greatest affinity for Ba-133 when the pH of the solution is between 4 and 8. (Figure 1)
The higher the salt content of the water, the slower the kinetics of Ba (or Ra) uptake. (Figure 2). The equilibrium is reached after 15 minutes of contact for samples with a salt content between 0 and 0.02%, whereas 25 minutes are required when the water contains 0.35% salt. For waters which composition is similar to seawater (3.5% salts), the equilibrium is reached in 90 minutes.
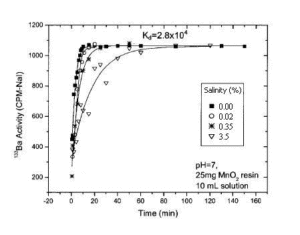
Figure 2 : Absorption kinetics of Ba-133 with respect to the salinity of the water sample. 10 mL of water sample traced with Ba-133 with 0%, 0.02%, 0.35% and 3.5% added salt. 25 mg MnO2 resin are added to pH 7 solutions under magnetic stirring for different times.
The batch uptake of radium and barium by the MnO2 Resin from deionized water and seawater have been studied and found comparable.
The recovery of Ba-133 and Ra-228 with respect to flow rate has been studied for 2 types of waters : deionized and artificial seawater. For salt water, the flow rate must not exceed 20mL/min, otherwise a loss in recovery of 30% may be observed. Additionally the chemical recoveries of Ba and Ra start to differ from each other, Ba-133 can thus no longer be considered as a chemical homologue, making it’s use as an internal standard undesirable.
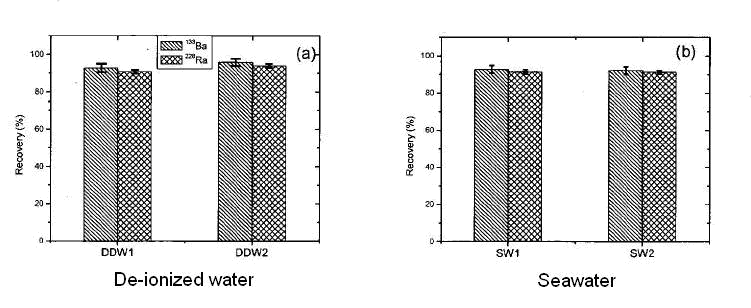
Figure 3 : Absorption recovery of Ba-133 and Ra-228. Duplicate experiment on deionized and artificial seawater. 1g/L of MnO2 resin.
The MnO2 resin is currently used with Ln Resin and DGA, Normal Resin in a method developed by Sherrod Maxwell [2] of Savannah River Nuclear Solutions.1.25 g/L of MnO2 resin is used to concentrate Ra/Ba from 1.0-1.5 liters of water. The sample is initially adjusted at pH 6-7 and 25 mg Ca are added per liter of sample. The sample is then loaded onto MnO2 Resin with a flow rate of about 15 mL/min. Ra is eluted with 15mL 4M HCl/1.5% H2O2. The Ra eluate is left for a minimum of 36 hours for Ac-228 ingrowth before being loaded stacked 2 mL cartridges of LN Resin (retention of U and Th) and DGA, Normal Resin (retention of Ac-228). Ac-228 is eluted from DGA resin with 10 mL 0.5M HCl, then micro-precipitated with CeF3 on Resolve™ Filter.
Bibliography
(1) Moon D.S., Burnett W.C., Nour S., Horwitz P., Bond A., Applied Rad. Isot.., 59, 255 (2003).
(2) Maxwell, S.L., presented at Eichrom’s North American Users’ Meeting, Oak Ridge, TN, May 3, 2005