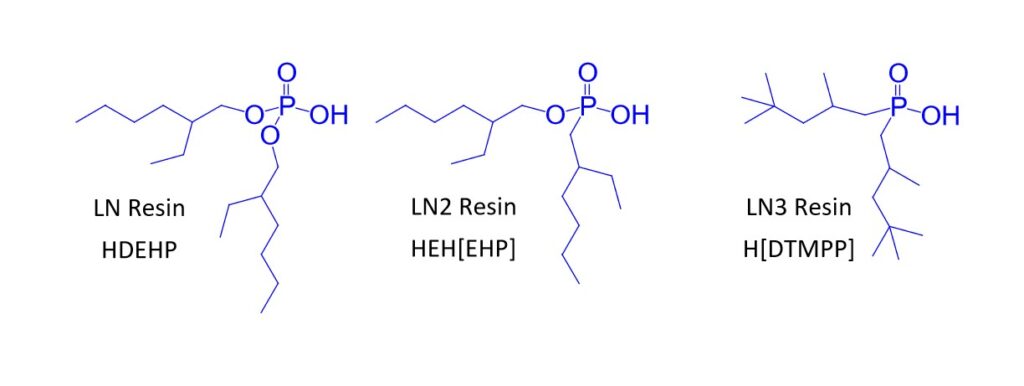
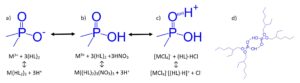
The LN Series resins (LN, LN2 and LN3) contain acidic akylphosphorus extractants on an inert polymeric support (Figure 1) and have been used in the purification of the nuclear medicine isotopes 86Y (1), 89Zr (2), 161Tb, and 177Lu (3) and various analytical applications (4-7).
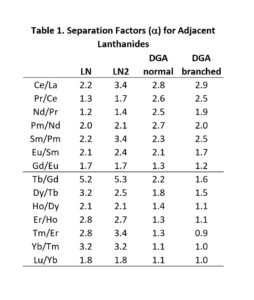
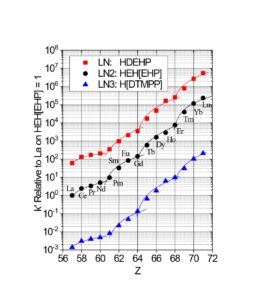
The LN series of resins is particularly useful for the separation of adjacent trivalent rare earth and actinide metal ions, exhibiting some of the highest separation factors of available chromatographic systems (Figure 2 and Table 1). LN and LN2 have been utilized in conjunction with DGA Resins for the production of high specific activity rare earth nuclides for nuclear medicine applications, including 177Lu and 161Tb (3).
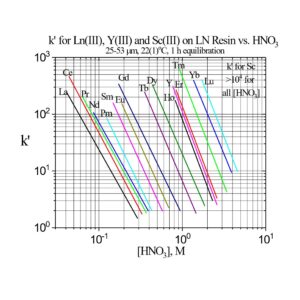
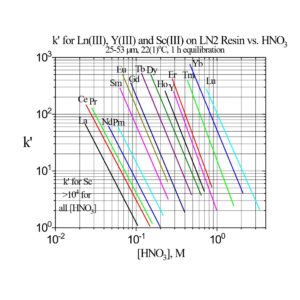
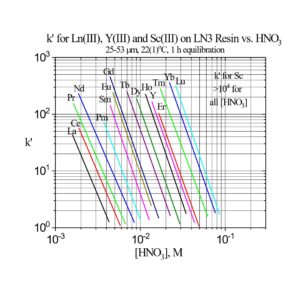
The separation factors for adjacent rare earths on LN and LN2 are similar (Table 1). However, the retention of metal ions at a given aqueous acidity decreases in the order LN > LN2 >> LN3, due to the decreasing acidity of the extractants as the P-OR bonds are replaced successively with P-R bonds (Figures 2-3) (8,9). The difference between the LN2 and LN3 is greater due to the additional affect of the increased steric hinderance for the 2,4,4-trimethyl-pentyl groups (LN3) vs 2-ethyl-1-hexyl groups (LN and LN2).
Plots of k’ for rare earths vs HNO3 for LN, LN2 and LN3 are presented in Figure 3. LN, with the highest k’ values, is useful for the separation of the light rare earths (La-Tb).
LN2 is often used for the separation of the heavier rare earths (Dy-Lu, Y and Sc), as these metal ions can be recovered from LN2 using more dilute acid than required for LN.
The retention of trivalent rare earths on LN3 requires very low acid concentrations, which are impractical for many separation methods. Therefore, LN3 is used primarily for the separation of higher valent metal ions such as Zr(IV) and U(IV), while rejecting most trivalent, divalent and monovalent metal ions.
The aqueous solubilities of the alkylphosphorus extractants used in the LN series resins increase as the mobile phase pH increases. Therefore, it is recommended that mobile phases used to load and elute the LN series resins be maintained below pH 3. Use of higher pH mobile phases can lead to significant loss of the extractant from the LN series resins.
As the acidity of the P-OH groups decrease, the equilibrium in Figure 4 shifts from (a) to (b) and from (b) to (c) more completely under acidic aqueous conditions. Extraction of metal ions from low acidity aqueous phases proceeds primarily through the cation exchange mechanism outline in Figure 4(a), where hydrogen bonded aggregates of the organophosphorus extractants, Figure 4(d), exchange a number of protons equal to the charge of the metal ion upon extraction. When operating under this extraction mechanism, log-log plots of the Dw (or k’ or D) vs the equilibrium aqueous acid activity will exhibit a negative slope equal to the charge of the metal ion in the extracted species (and the number of protons exchanged).
As the concentration of acid in the aqueous phase increases, the equilibrium shifts from 4(a) to 4(b), where the P-OH groups are fully protonated. Under these conditions, most trivalent rare earths and actinides and lower valence metal ions are poorly extracted. However, the extraction of tetravalent and hexavalent actinides remains high due to extraction via a neutral (solvating) extraction mechanism, with the metal ion charge neutralized by coextraction of nitrate or chloride ions.
At very high acidities, the P=O groups of the organophosphorus extractants extract a significant amount of acid, forming P=O—H+NO3– or P=O—H+Cl–. These acid adducts can act as weak base anion exchangers, extracting anionic metal complexes and exchanging NO3– or Cl–, Figure 4(c).
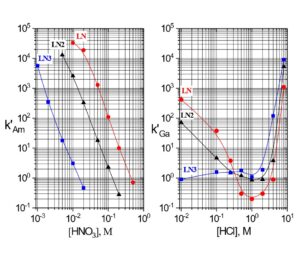
Examples of the three extraction mechanisms are provided in Figure 5. From HNO3, the extraction of Am(III) by the LN series is highest at low acidity and decreases with a log-log slope of -3 as the HNO3 concentration in the aqueous phase is increased. The magnitude of the Am(III) extraction at a given acidity decreases in the order LN > LN2 >> LN3 as the acidity of the P-OH groups decreases in the same order. For Ga(III) extraction from HCl, the log-log plots of k’ Ga vs HCl concentration exhibit similar behavior to Am(III) at low acidity. As the P-OH groups become protonated at higher HCl concentrations, the Ga(III) extraction becomes very low, as GaCl3 is poorly extracted by the protonated neutral extractants. At the highest acidities, Ga extraction increases, as the P=O groups are protonated and [GaCl4]– is extracted via the weak base anion exchange mechanism in Figure 4(c).
DGA resins are often used in conjunction with LN and LN2 for the separation of rare earth elements. The LN series and DGA resins complement each other, as the LN resins are typically loaded in dilute acid and stripped with more concentrated acid, while DGA resins are loaded in more concentrated acid and stripped with dilute HCl. Therefore, a number of alternating LN series and DGA resins may be used in sequence, without evaporation of eluates between columns.
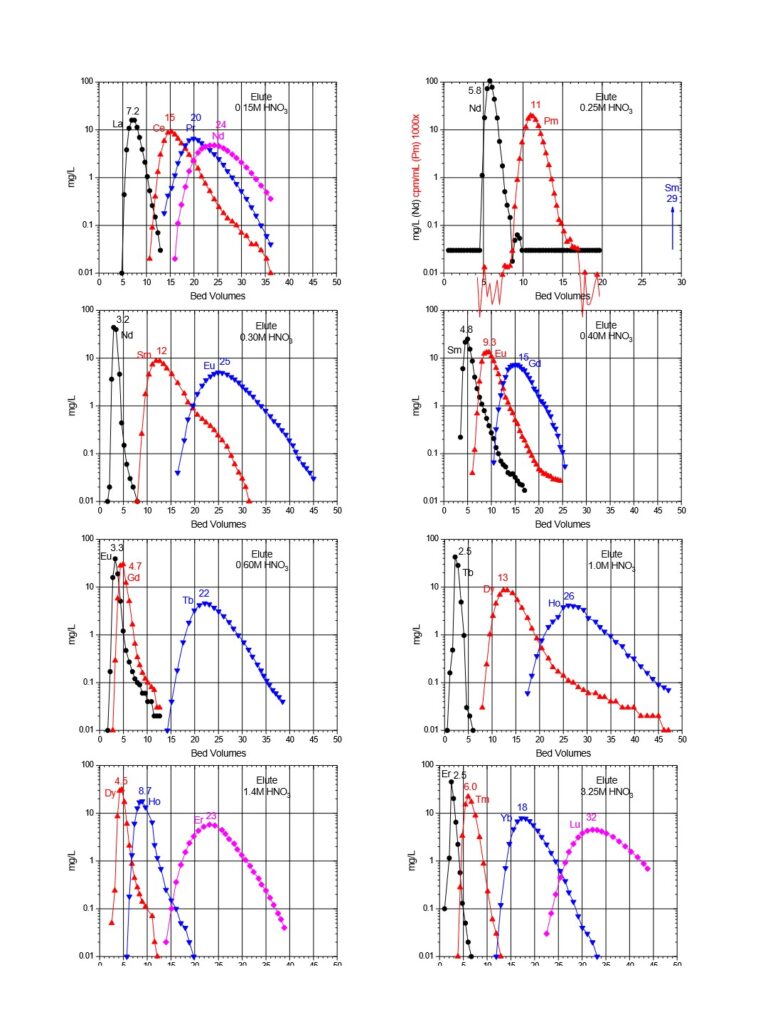
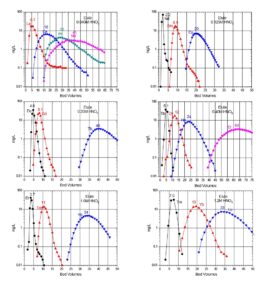
Typical elution curves for all the rare earth elements on LN and LN2 resins are presented in Figures 6 and 7. Similar curves for the DGA resins can also be found on the DGA resin webpage.
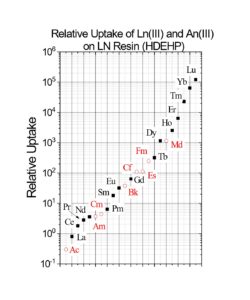
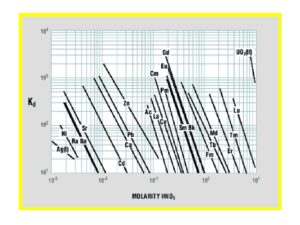
Early work by Horwitz et al. (10) , provided distribution coefficients (Kd) versus nitric acid for various metal ions on LN resin. This data is reproduced in Figure 8 and has been used as the starting point for a number of radiochemical separations in use today. Additional data for the retention of metal ions on LN, LN2 and LN3 is compiled in Figures 9 and 10 (8). Note that for LN resin, Kd can be converted into k’ (an approximation of free column volumes to peak maximum) by dividing by 4.33. LN resin has been applied to the analysis of radium, neodymium, and promethium. As the data in Figures 8-10 indicate, many more analytical applications are possible.
Bill Burnett et al. (4) developed an analysis procedure for 228Ra using LN resin, which selectively extracts 228Ac, the direct beta particle-emitting daughter of 228Ra. LN resin employs the same HDEHP extractant used in EPA Method Ra-05-1 (EPA Procedures Manual 520/ 5-84-006) without the generation of mixed organic waste.
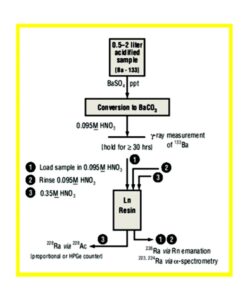
An outline of Bill Burnett’s method is provided in Figure 11. After a barium sulfate precipitation to concentrate Ra and metathesis to barium carbonate, the sample is dissolved in 10 mL 0.095M HNO3 , stored > 30 hours for 228Ac ingrowth, and loaded onto a prepackaged LN resin column (0.65 g). The column is rinsed with 15 mL of 0.095M HNO3 to remove alkaline earths, lead and other interferences, and the load and rinse fractions are collected and analyzed for 226Ra by radon emanation. Finally, 10 mL of 0.35M HNO3 is used to quantitatively elute actinium from the LN resin, and an 228Ac source is prepared for gas flow proportional counting. For routine work, a minimum detectable activity (MDA) of <1.0 pCi/L (0.037 Bq/L) is achieved for a 2L sample with 80% barium recovery with a 30 minute count.
This method was used to analyze samples from a US-EPA inter-laboratory comparison for a number of 228Ra samples. All results agreed with the expected values at the 95% (2 sigma) confidence interval. Additional development of this method at Eichrom has resulted in Eichrom Method RAWO3, where the BaSO4 precipitation/carbonate metathesis steps have been replaced with a cation exchange concentration step. Alpha spectrometry is used for the analysis of 226Ra as well has the shorter-lived 224Ra. Additional performance data was presented at the Eichrom North American Users’ Meeting in May 2002.
LN resin is also useful in the analysis of 147Pm (5,6). In analysis of environmental and waste samples from nuclear facilities, it is necessary to separate promethium from other fission products and the actinides to obtain an accurate measurement. A method for the measurement of 147Pm water is outlined by Cable, et al. (6), where a 0.5-2 L water sample is concentrated by a calcium phosphate precipitation or evaporation. The resulting residue is dissolved in 0.2M HNO3 and ascorbic acid , which reduces Fe(III) to Fe(II). The sample in 0.2M HNO3 is loaded onto a standard prepackaged LN resin column, and rinsing with 0.2M HNO3 removes americium, strontium, barium, radium, cesium, Fe(II) and many other interferences. Promethium is retained along with bismuth, yttrium and the potential tracers samarium or gadolinium. Fe(III) would also be strongly retained by the LN resin, however, a test with 55 mg Fe resulted in no breakthrough of Pm from the LN resin column, demonstrating the effectiveness of the ascorbic acid reduction of Fe(III) to Fe(II).
The promethium, along with the samarium or gadolinium tracer, is eluted with 5 mL of 1 M HNO3. Corrected recoveries of 147Pm were >88% using 148Gd as a yield monitor. Excellent decontamination was achieved from 60Co, 134Cs/137Cs and 89Sr/90Sr. One other potential interference is 144Ce, a beta and gamma emitter. A check with gamma spectroscopy should be used to eliminate this as a potential false positive.
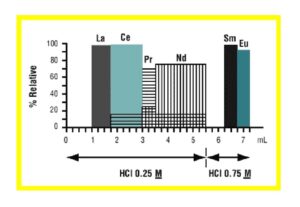
Christian Pin et al. (7) reported a method for the sequential separation of Sm, Nd, Th and U in silicate rocks. The method uses Eichrom TRU resin in series with LN resin. For samples high in iron, such as basaltic samples, a 50WX4 strong acid cation exchange column (available from Eichrom) is also used for up front removal of Fe. After dissolution and possible treatment with cation exchange resin, the sample is loaded onto a TRU resin column in 1M HNO3. Rinsing the TRU resin with 1M HNO3 removes many impurities, and the light rare earth elements (LREE) can then be stripped with 0.05M HNO3 . The LREE fraction from the TRU resin column is loaded directly onto an LN resin column, and the LREE are retained. Figure 12 shows the elution of La, Ce, Pr, Nd, Sm and Eu on a 0.3 g column of LN resin (50-100 mm). A total of 5.5 mL of 0.25M HCl was used to strip the La, Ce, Pr and Nd with no detectable Sm (ID-TIMS). Sm and Eu were then collected by elution with 0.75M HCl. Comparison with 15 international standard reference materials of silicate rocks showed good agreement.
LN resin has a calculated maximum capacity of approximately 22 mg Nd/mL of resin. However, in most analytical procedures, it is recommended not to exceed 10-20% of this capacity or 2-4 mg/mL resin or 4-8 mg/2 mL prepacked column or cartridge. Additional physical properties for the LN series resins are presented in Table 2.
LN resin is manufactured in three particle sizes (25-50 µm, 50-100 µm, and 100-150 µm) and is sold in bottles or ready to use in prepackaged columns (for gravity flow) and cartridges ( for vacuum assisted flow.)
References
1) Eichrom Application Note AN-1623, “Separation of 86Y from Strontium Target”
2) Eichrom Application Note AN-1622, “Separation of 89Zr from Yttrium Target with LN3 Resin”
3) E. P. Horwitz, D. R. McAlister, A. H. Bond, R. E. Barrans, J. M. Williamson, “A Process for the Separation of 177Lu from Neutron Irradiated 176Yb Targets,” Applied Radiation and Isotopes, 63, 23-36 (2005).
4) Burnett, W., Cable, P., Moser, R. Determination of Radium-228 in Natural Waters Using Extraction Chromatographic Resins, Radioactivity & Radiochemistry, Vol. 6, No. 3, pp. 36-44, (1995).
5) Pm Separation via Ln Resin-A Progress Report, 41st Annual Conference On Bioassay, Analytical & Environmental Radiochemistry (Eichrom Workshop). Boston, MA, (1995).
6) Burnett, W.; Cable, P.; Wong, R.; Corbett, D.R.; Schultz, M.; Stewart, B.; Westmoreland, J. Pm/Sm Separation via Ln Resin, Eichrom Atlanta Users’ Seminar, Atlanta, GA, (1996).
7) Pin, C., et al. Sequential Separation of Light Rare-Earth Elements, Thorium and Uranium by Miniaturized Extraction Chromatography: Application to Isotopic Analyses of Silicate Rocks, Analytica Chimica Acta,Vol. 339, pp. 79-89, (1996).
8) D. R. McAlister, E. P. Horwitz, “The Characterization and Novel Applications of Extraction Chromatographic Materials Containing Bis(2-ethyl-1-hexyl)phosphoric Acid, 2-ethyl-1-hexylphosphonic acid, mono 2-ethyl-1-hexyl ester and 2,4,4-trimethyl-1-pentylphosphinic acid,” Solv. Extr. Ion Exch., 25(6), 757-769 (2007).
9) E. P. Horwitz, D. R. McAlister, M. L. Dietz, “Extraction chromatography versus solvent extraction: How similar are they?” Sep. Sci. and Technol., 41(10), 2163-2182 (2006).
10) Horwitz, E.P., Bloomquist, C.A.A., Chemical separations for super-heavy element searches in irradiated uranium targets. J. Inorg. Nucl. Chem. 37, 425–434 (1975).
Additional LN Series Applications
AN-1417 Rapid Determination of 226/228Ra in Water Samples
AN-1811 Separation of Ce from Rare Earth Nitrates