CL Resin, used for the separation of chloride and iodide, is based on an extractant selective for the platinum group metals, gold and silver. The selectivity for halides is introduced by loading the resin with silver(I) ions.
DW values of selected cations on the CL Resin are shown in Table 1. For practical reasons, sulfuric acid was chosen as extraction medium. The CL Resin has high selectivity for Pd(II) and Ag(I), whereas the DW values of the other tested metal ions are low. DW values for Ag(I) remain high over a wide pH range (from 1M H2SO4 to dilute sulfuric acid, pH 5). Ag(I) is therefore extracted and remains fixed on the resin over a wide range of pH values.
| Analyte | Extraction condition | DW, mL.g-1 |
| Ag(I) | 1M H2SO4 | 650000 |
| Ag(I) | Sulfuric acid, pH 3 | 600000 |
| Ag(I) | Sulfuric acid, pH 5 | 350000 |
| Cd(II) | 1M H2SO4 | <1 |
| Ce(III) | 1M H2SO4 | 4 |
| Co(II) | 1M H2SO4 | <1 |
| Cu(II) | 1M H2SO4 | <1 |
| Fe(II) | 1M H2SO4 | <1 |
| Mn(II) | 1M H2SO4 | <1 |
| Ni(II) | 1M H2SO4 | <1 |
| Pd(II) | 1M H2SO4 | 87000 |
| Zn(II) | 1M H2SO4 | 25 |
Table 1: DW values CL Resin of selected cations in sulfuric acid.
The loading of the CL Resin with silver cations provides the mechanism for selectivity of halides which form sparingly soluble complexes with Ag(I). DW values for chloride and iodide on the silver loaded CL Resin in 1M H2SO4 were determined to be 1600 and 1980, respectively. Iodine and chloride are thus well retained under those conditions. The CL Resin used for the DW experiments was loaded with 20 mg Ag(I) per gram of CL Resin prior to the extraction experiments, which corresponds to a typical working capacity for the CL Resin. The capacities for chloride and iodide of the silver loaded resin under these conditions are: 16.3 +/- 1.6 mg iodide per 2 mL column (approx. 25 mg iodide per g resin) and 4.3 +/- 0.2 mg chloride per 2 mL column (approx. 6.5 mg chloride per g resin). Higher capacities for halides may be obtained by increasing the silver load of the CL Resin.
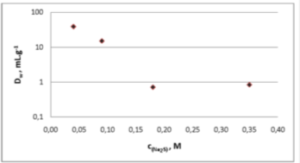
Figure 2. Dw Cl- and I- on Ag+ loaded CL Resin at pH 7 from varying concentrations of Na2S.
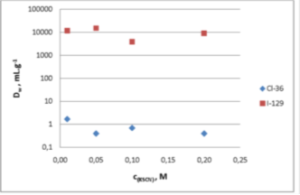
In order to evaluate best suited conditions for the separation of chloride and iodide, DW values of chloride and iodide were determined on silver loaded CL Resin in varying SCN- and S2- concentrations: Fig. 1 and 2 show the obtained results.
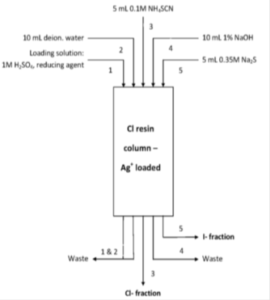
Chloride can be easily eluted from the CL Resin using SCN- solutions, while iodide remains fixed. Iodide can then be eluted from the CL Resin using a high concentration solution of S2-. Based on this information, a method for the separation of chloride and iodide was developed and optimized by Zulauf et al. [1] (Figure 3) In order to assure that both chlorine and iodine are present as chloride and iodide, the sample should be loaded from a sulphuric acid solution containing 0.1M SnSO4 as reducing agent. This is especially important in the case of chlorine since chlorate is not fixed on the resin, while iodate is extracted, as could be expected from silver salt solubility data.
Figure 3: Separation of chloride and iodide on silver loaded CL Resin.
The sample is preferably loaded onto the silver loaded CL Resin from 1M H2SO4 (slightly acidic or even neutral conditions are also acceptable). Rinsing with deionized water removes many matrix elements and potential interferents from the column. Chloride is then eluted in a small volume of NH4SCN or NaSCN.
During method optimization it was shown that rinsing the column with a dilute alkaline solution before iodide elution lead to a strong increase of the iodide yield. Therefore, the CL Resin column is rinsed with 1% NaOH before iodide is finally eluted in a small volume of a Na2S solution. (Note: all work with the Na2S solutions should be performed under a fume hood, including the addition of the liquid scintillation cocktail.).
The small elution volumes used for elution allow for direct measurement of the obtained fractions by LSC (Remark: some LSC cocktails reduce traces of Ag+ co-eluted from the column resulting in blackened LSC samples; it is thus advisable to test your cocktail before use).
CL Resin is manufactured in two particle sizes (50-100μm, and 100-150μm) and is sold in bottles or ready to use in prepackaged columns (for gravity flow).
Source for all published data:
(1) A. Zulauf, S. Happel, M. B. Mokili, A. Bombard, H. Jungclas: Characterization of an extraction chromatographic resin for the separation and determination of 36Cl and 129I. J. Radanal Nucl Chem, 286(2), 539-546 (2010).
| Particle Size | Bottles | Part Number |
| 100-150 μm | 25 grams | CL-B25-A |
| 50 grams | CL-B50-A | |
| 100 grams | CL-B100-A | |
| 200 grams | CL-B200-A | |
| Columns (2mL) | Part Number | |
| Package of 20 | CL-C20-A | |
| Package of 50 | CL-C50-A | |
| Package of 200 | CL-C200-A |
| Particle Size | Bottles | Part Number |
| 50-100 μm | 25 grams | CL-B25-S |
| 50 grams | CL-B50-S | |
| 100 grams | CL-B100-S | |
| 200 grams | CL-B200-S |