Eichrom’s SCX (strong cation exchange) silica contains sulfonic acid groups chemically bonded to irregular silica. The SCX silica is thoroughly purified to remove metal ion contamination to improve radiolabeling of purified radionuclides in nuclear medicine applications.
Specifications
substrate: irregular silica
particle size: 35-60 µm
porosity: 60Å,
surface area: 450 m2 /g
pH stability: 2-10
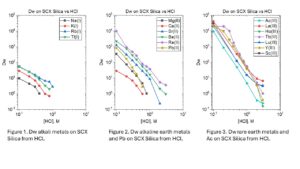
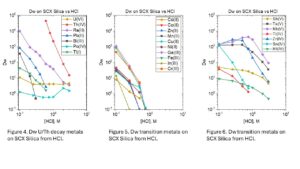
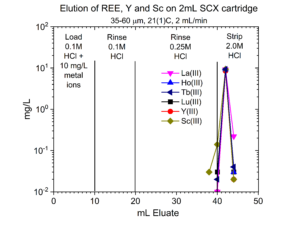
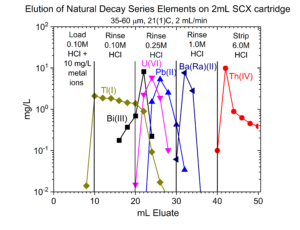
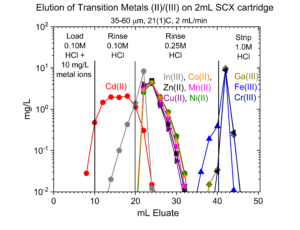
Dry weight distribution ratios (Dw) for selected metal ions on the SCX-silica vs HCl are provided in figures 1-6.
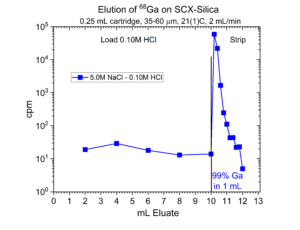
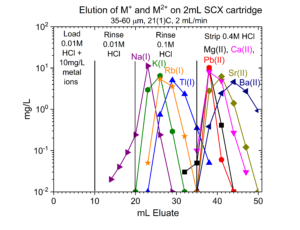
One application of the SCX-silica is the purification of 68Ga. The 68Ga is produced by elution from a generator column containing its parent 68Ge with 0.1M HCl. The 68Ga is trapped on the SCX-silica from the 0.1M HCl, while 68Ge and many stable metals pass through. The 68Ga is then recovered using a small volume of 5M NaCl – 0.1M HCl.[1] Elution curves for 68Ga (Figure 7) and many other stable metals (Figures 8 – 11) on SCX-silica are presented below.
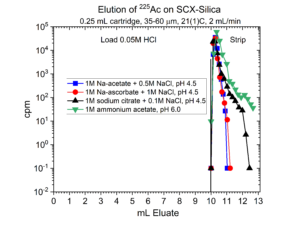
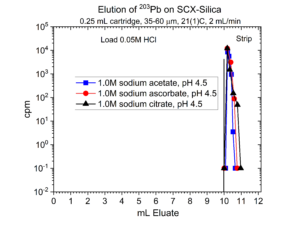
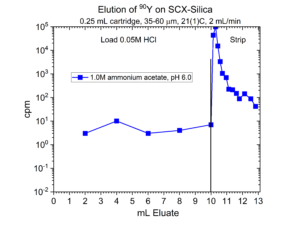
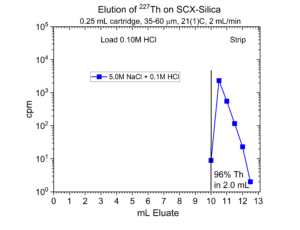
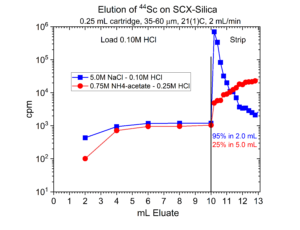
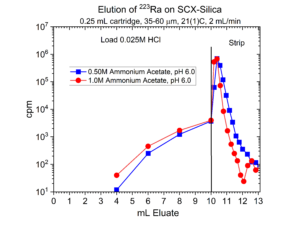
Many radionuclides used in nuclear medicine applications are received in mineral acid solutions, such as dilute HCl. Prior to radiolabeling of the metal ions to biolocalization agents through chelation, it is often necessary to convert the matrix of the radionuclides into a biocompatible buffer such as sodium acetate or ammonium acetate with a pH of 4.5 – 6.5. Another application of SCX-silica is the concentration of radionuclides from dilute acid, such as 0.01 – 0.10M HCl, and then elution from SCX-silica with a buffer solution.[2,3] Examples of this application for 225Ac, 203Pb, 90Y, 227Th, 44Sc, and 223Ra are presented in figures 12-17.
References:
[1] Schultz, M. K.; Mueller, D.; Baum, R. P.; Leonard Watkins, G.; Breeman, W. A. P. A New Automated NaCl Based Robust Method for Routine Production of Gallium-68 Labeled Peptides. Applied Radiation and Isotopes 2013, 76, 46–54. https://doi.org/10.1016/j.apradiso.2012.08.011.
[2] M.A. Eddy, E. Rush, D. R. McAlister, “Recovery of Select f-elements, Y and Sc from chromatographic resins in an acetate buffer,” Solv. Extr. Ion Exch., submitted (2025).
[3] van der Meulen, N. P.; Bunka, M.; Domnanich, K. A.; Müller, C.; Haller, S.; Vermeulen, C.; Türler, A.; Schibli, R. Cyclotron Production of 44Sc: From Bench to Bedside. Nucl Med Biol 2015, 42 (9), 745–751. https://doi.org/10.1016/j.nucmedbio.2015.05.005.